Most Hydrophobic Columns
The following columns (in alphabetical order) show a pronounced hydrophobic character. Several criteria were considered such as selectivity factors, behaviour towards polar/non-polar analytes, bonding density, homogeneity of the surface, etc. Example:
On CORTEX C18, a highly hydrophobic material, the peak sequence is ethylbenzene/fluorenone, on Synergi POLAR RP, a highly polar material exactly the opposite, fluorenone/ethylbenzene.
XBridge C18/ UPLC BEH C18
Most Polar Columns
The following columns (in alphabetical order) show a pronounced polar character. Several criteria were considered such as selectivity factors, behaviour towards polar/non-polar analytes, bonding density, homogeneity of the surface, etc.
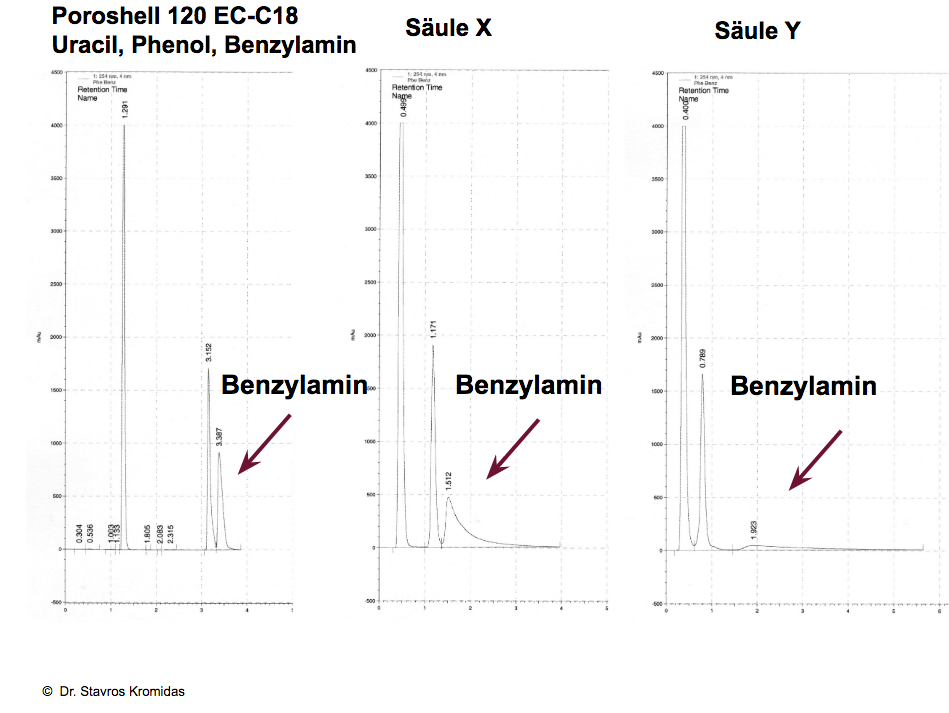
Peak shape benzylamine
The alpha value Benz/Phe (benzylamine/phenol) is to be regarded as a measure of the ion exchange/hydrogen bridge bond ratio. Furthermore, strong tailing and a long retention time or even irreversible adsorption of the strong base benzylamine (alpha value Benz/Phe nominally greater than 20) indicates the tendency of the corresponding stationary phase to strong cation exchange interactions, thus it exhibits a strong polar character. The peak shape of benzylamine ranges from a non-evaluable peak to a fairly symmetrical peak, see example below.
On the following 12 columns (in alphabetical order) the peak shape of benzylamine is good: There are no (accessible) acidic silanol groups on the surface and thus hardly any cation exchange interactions take place, the result is: Symmetrical peaks even with strong bases. Note: The benzylamine test is a very strict test.
XBridge C18/UPLC BEH C18