SIMPLIFIED RULES FOR COLUMN AND SOLVENT SELECTION
The silica gel matrix and the particle size
heavily contaminated sample, difficult matrix e.g. biological material, environmental sample, plant extract, food, animal feed, starch, polymer matrix etc.? Think first of monoliths (e.g. Chromolith Performance/Onyx). If it is to be a porous material, then tend towards one with a large specific surface area (> 350 m 2 /g). Furthermore, is gradient elution planned, as more than approx. 10-15 peaks are expected? If both questions (difficult matrix, gradient) are to be answered with "yes": with a focus on a long column life, in the case of a porous material 5 µm would be preferable; only if thorough sample preparation can be guaranteed in routine operation could you also possibly think about 3-3.5 µm material or smaller or of Core Shell phases.
The column dimensions
- Do you expect approx. ≤ 10 peaks and do you also plan gradient elution? Column length: Rather 50 mm, never longer than 100 mm (for 20-25 peaks
125-150 mm) - Do you expect less than about 10 peaks and should it be a robust method for routine use? Probably in this case you intend to establish an isocratic method - take a 100 mm column
- Do you expect low concentrations? Think of a 3 mm column. You don’t have a difficult matrix and the pressure does not bother you either? Then you should think of a 2.1 mm column.
The stationary phase
Does the sample contain the following substances?
- pronounced polar components such as dipoles, strong acids/bases, halogenated molecules, dyes, flavonoids, carotenoids, native acids, amino-sugars, phospholipids, metabolites, (partly) ionic species, zwitterions
- sterically demanding structures (e.g. twisted molecules, steroids)
- unsubstituted polynuclear aromatics
- "difficult" isomers (e.g. positional, structural or double bond isomers)
- chemically very similar molecules...
... then more suitable are polar stationary phases, e.g. silanol-containing materials, PFP (pentafluorophenyl), biphenyl, mixed-mode, EPG (embedded polar group) phases. However, you would have to accept problems regarding reliability in everyday life (batch reproducibility, difficulties with validation, etc.). Note: the reference "polar stationary phases" aims at the highest possible selectivity; this in no way means that with hydrophobic materials a satisfactory selectivity for polar components, sufficient for the given purpose, would not be possible.
For all other cases such as
- non-polar components, (e.g. there is a minor difference in hydrophobicity)
- non-polar and polar components (e.g. active substance/metabolite, ∆ number of CH2 groups, CH3 vs. OH group)
- saturated aromatics, no heteroatom
- non-ionic, fairly weak polar components (e.g. aldehydes, ketones or also weak acids)
…are hydrophobic phases more suitable, with acetonitrile as organic solvent.
The solvent
Methanol often shows better selectivity in such cases than acetonitrile, for example π-π interactions are suppressed in acetonitrile.
EXTENSIVE EXPLANATION
Summary of key statements
Firstly a summary of key statements:
- With acetonitrile and buffer - especially in alkaline – you do a kind of "levelling out", the individual differences of the stationary phases regarding selectivity are hardly noticeable
- "Polar components and/or similar structures? Use a polar column plus methanol (plus low temperature, e.g. 10-15 °C)"
- "With methanol, the columns show a much wider range of selectivity”
- "If (additional) polar interactions are possible, the individual differences of the stationary phases with regard to selectivity take effect". This is the case if the analyte has a pronounced polar character or is present in a (partially) ionic form. With regard to stationary phases, this means an (additional) polar group. This can be a silanol group or a functional polar group such as EPG, PFP, biphenyl, amide/carbamate (EPG) or mixed mode phase.
- "Good aromatic selectivity is shown by stationary phases which either have a polymer/polymerized layer on the surface or which above all are capable of polar interactions"
- "In the case of unknown samples, testing with truly orthogonal columns is advisable". And "It is advantageous to enable several interaction mechanisms at the same time!"
- "Alternative strategy when a column changer is not used: "Good", classical, C18 column plus subsequent tests using one or more "completely" different column(s) to confirm selectivity"
Methanol vs acetonitrile
"With acetonitrile and buffers, differences fade into the background."
At a certain pH value of the eluent acidic or basic molecules are neutralized, the buffer perhaps being used ensures a constant pH value. Some or all of the components are therefore present in a neutral form, so with an RP material there are predominantly neutral interactions. Now, it is true that neutral, hydrophobic, in other words typical RP interactions are not particularly characteristic, the result being that individual differences of the components (e.g. differences in the ionic character) fade into the background. This also applies to the stationary RP phases, additional polar groups (silanol groups, other polar groups) which may be present – and which are responsible for the differences between the phases - can hardly come into action against neutral species. This effect is much more pronounced with acetonitrile in the eluent than with methanol. Simplified conclusion: With acetonitrile and buffer - especially in alkaline – you do a kind of "levelling out"; the individual differences of the stationary phases regarding selectivity are hardly noticeable. This is the main reason why when using acetonitrile and buffer, different columns often produce very similar chromatograms, see the following two examples: although the four Waters columns used here do have different properties, the chromatograms are quite similar. This applies both to the separation of bases and acids.
The facts described here also apply to unbuffered systems, see further below.
"Methanol: advantages in the case of polar components and polar phases"
Even in a deliberately unfavourable constellation (strong bases and acidic buffer) methanol proves to be advantageous: 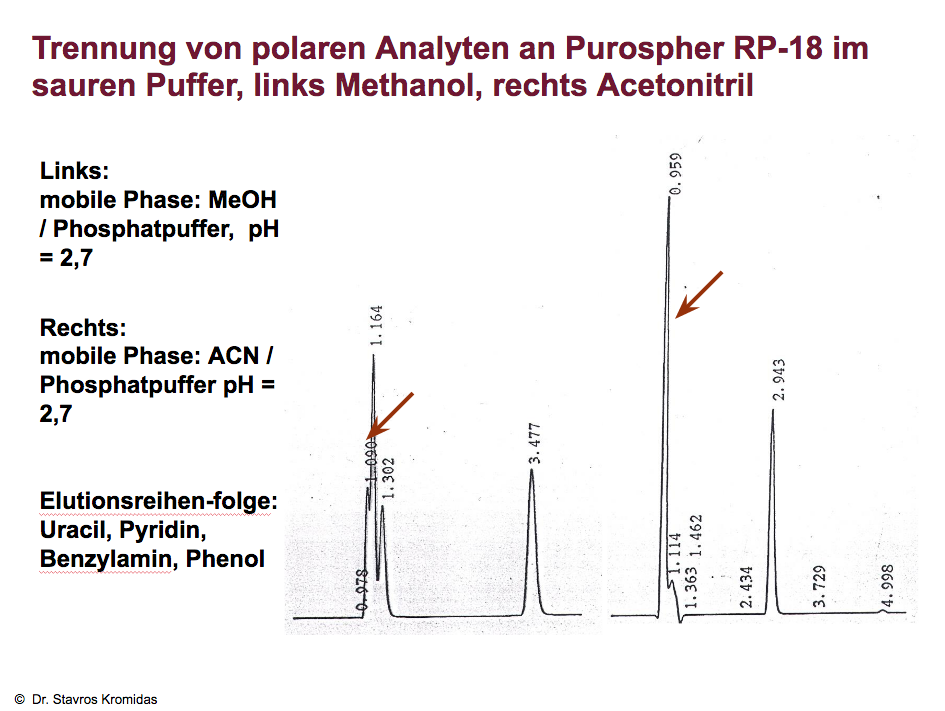
Despite the "wrong" pH value, the peaks are at least partly separated (left chromatogram), with acetonitrile as organic solvent only one peak is obtained (right chromatogram). Also in the case of strong acids is the selectivity with methanol better than with acetonitrile (left vs right chromatogram).
In the case of molecules with similar structure (isomers, phospholipids, steroids, etc.), polar interactions are advantageous for the separation, i.e. columns with a polar character are required. Furthermore, also in this case methanol often shows a better selectivity for polar components compared to acetonitrile. Here an example: a mixture of uracil, aniline, phenol, o-, m-, p-toluidine, ethylbenzene and fluorenone was injected onto different columns at 60/40 methanol/water. Classic, endcapped C18 phases cannot offer polar interactions to the three positional isomers, as a result only two peaks are obtained for the three isomers, and the chromatograms with such columns look almost identical.


As soon as polar interactions are possible (silanol groups, PFP group), the selectivity for the separation of the toluidine’s is improved, note also the elution reversal with ethylbenzene/fluorenone (blue arrow Fig. 8), see also further below. However, additional polar interactions often lead to a deterioration of the peak shape. A simplified recommendation: "Polar components and/or similar structures? Use a polar column plus methanol (plus low temperature, e.g. 10-15 °C) for good selectivity and be willing to compromise on peak shape and retention time as well as column stability and batch reproducibility".
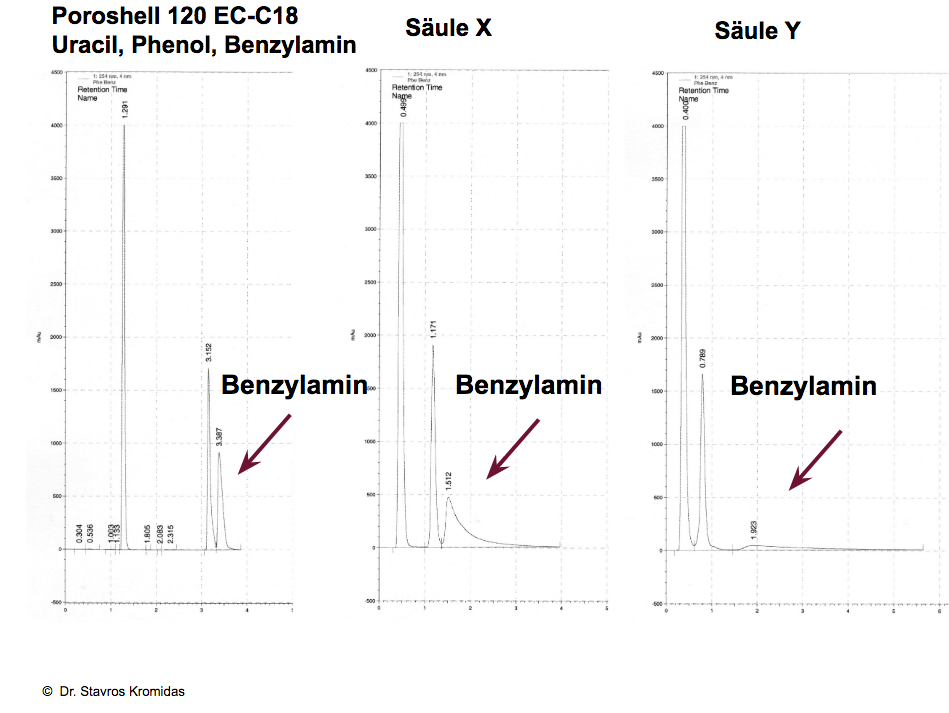
Two more examples:
Here the separation of polar components is shown, left with methanol, right with acetonitrile. Findings: With methanol a very polar component is almost baseline separated from the dead time peak (uracil), also phenol and benzylamine are well separated. With acetonitrile, the polar impurity at the dead time peak is just hinted at as a disturbance, phenol and benzylamine coelute.
In this diagram the alpha values for the separation of two isomers, 3- and 4-nitroaniline, are shown on various columns, blue in acetonitrile, red in methanol. Findings: The alpha values in acetonitrile range between approx. 1.0 and 1.35, in methanol between approx. 0.85 (elution reversal) and 1.45. This means: "With methanol the columns show a much greater range of selectivity".
Polar and non-polar interactions
As noted above, neutral interactions are not particularly characteristic. Such effects occur in the case of neutral components or components neutralized via the pH value. In such cases, the stationary phases can hardly unfold their individuality with regard to selectivity, three examples illustrate this:
In this diagram, the bars represent the alpha values for the separation of ethylbenzene/toluene, a typical RP analyte pair (methylene group selectivity, difference only one CH2 group) on various columns. The alpha values on quite different columns - e.g. Symmetry C18 and Platinum EPS - are very similar; overall the values range between 1.30 and 1.45. If on the other hand polar interactions are also possible, e.g. simply by an additional oxygen in the separation of fluorene/fluorenone (link bar chart), then alpha values between 1.5 and 3.4 result. Also in the separation of ethylbenzene/fluorenone (link bar chart), due to additional polar interactions the alpha values range between approx. 0.45 (elution reversal) and 1.75. One can state as follows: "If (additional) polar interactions are possible, the individual differences of the stationary phases with regard to selectivity take effect; otherwise the chromatograms look quite similar".
In simple terms: "Same with the same", e.g. strong acids are present in the (partly) ionic form, for good selectivity stationary phases with a polar character are required, see for example the following result
The strong acids phthalic/terephthalic acid are better separated on the polar XBridge Shield material than the two weak acids 3-/4-hydroxybenzoic acid (alpha value blue bars). On the other hand, the 3-/4-hydroxybenzoic acid pair is better separated on the hydrophobic XBridge C18 material (alpha value red bars).
"Good aromatic selectivity is shown by stationary phases which have either a polymer/polymerized layer on the surface or which above all are capable of polar interactions", see diagram). The polar version of Acclaim (Acclaim PA C16) shows a better selectivity for the separation of chrysene/perylene compared to the hydrophobic Acclaim C18; the yellow line corresponds to the alpha values. Furthermore, in no case does stronger interaction mean better selectivity. Aromatic compounds make up a large part of the components to be separated in HPLC. With the help of selectivity web charts (link to D FAQ 4), the rules presented here for the separation of polar and non-polar aromatic compounds can be exemplarily demonstrated.
How do the characteristics of the phase surface influence selectivity?
Phases showing good hydrophobic selectivity are located in the upper part of the "central selectivity map" (large alpha value ethylbenzene/fluorenone). These are phases that have the following characteristics: high loading (roughly about > 17-18 % C), and/or "polymer bonding", furthermore hardly any active (= acidic, dissociated) silanol groups - these are the "good", classic C18 phases. With strongly polar phases such as mixed-mode, biphenyl or PFP phases, elution reversal occurs, the selectivity - with negative alpha values! - is also very good
A distinction should be made here as follows:
Silanophilic phases: phases having dissociated silanol groups. These are, for example, older, non-endcapped materials, which are strongly silanophilic, e.g. Zorbax ODS, Hypersil ODS, Spherisorb ODS 1, Bondapak, LiChrosorb, LiChrospher/Superspher. With basic samples the silanol activity is particularly pronounced in the neutral/acidic range: very strong interactions with protonated N-containing molecules - up to irreversible sorption on the surface. As a rule, there is good selectivity for strongly polar molecules with, however, often strongly asymmetrical peak shapes. If the molecules are not present in ionic form, that is if ion exchange interactions are not possible, then the other characteristics of the phase in question come to the fore. For example, Zorbax ODS, due to its high carbon content, also exhibits good hydrophobic selectivity. In contrast, a number of more modern, thoroughly endcapped phases hardly show any silanol activity even in the very strict benzylamine test (Test 6 No. 8), even strong bases elute with a good peak shape, e.g. PoroShell 120 EC C18, PoroShell 120 HPH C18, Cortecs C18, XSelect HSS C18, XBridge C18, YMC Pro C18 RS (Link 13, 14, Link peak shape benzylamine).
- Summarised: If ionic/strongly polar interactions are necessary for the separation, you can still think of older materials, see above. Of course also of modern non-endcapped materials such as Poroshell 120 SB C18 or on PFP columns or mixed-mode materials like Primesep A or Obelisc R. Long retention times, very high pH-value dependence and possibly tailing might have to be accepted. Is on the other hand the selectivity given by classical RP interactions (confirmation by orthogonal tests!)? Then this probably means that the hydrophobic character of the analytes is quite pronounced. In this case, materials listed as examples in the second row would certainly be a good choice. With satisfactory selectivity, one can expect a very good peak shape and a long column life. Usually and wisely, acetonitrile is used in such cases.
- Undissociated silanol and other polar groups; phases with a polar character are required for the separation of polar molecules. These phases are located on the right-hand side of the selectivity map "central selectivity map" (large alpha- value Tri/o-Ter). A particularly good steric selectivity - e.g. separation of isomers - can be found in phases with a PFP (pentafluorophenyl) or EPG (Embedded Polar Group) group or in phases with a high density and/or "polymer bonding".
Summarised: For a good polar or a good steric selectivity, phases on the left-hand side of the "central selectivity map" are less suitable. Should one nevertheless decide for whatever reasons for a phase from this area, then a phase with free silanol groups would be preferable to an extremely well covered, hydrophobic, endcapped phase. The chromatograms with the largest differences are obtained (in other words the different properties of the phases become noticeable) when first of all ionic interactions play an important role with regard to selectivity. In second place comes steric selectivity, here too the differences between the phases appear, see also further below. Finally, the choice of the stationary phase in the case of weak, undifferentiated, non-polar interactions is relatively unimportant; the required selectivity can sooner be achieved, if necessary, via the mobile phase.
Hardly any information about the sample?
- "Large column diversity necessary for testing! “
With unknown samples, it is time-saving to find the suitable column by using a column changer. In such cases, with the help of for example the selectivity maps or bar charts truly different columns should be tested - not just simply several C18 columns. This applies not only to the chemical character of the stationary phase but also to the pore diameter.
Three examples are given below:
- On the two C18 columns LiChrosorb and Reprosil AQ, with pore diameters of 100 Å and 120 Å respectively, five main peaks appear; with Jupiter, also a C18 material but with a pore diameter of 300 Å six main peaks

Such steric effects are therefore observed not only with large molecules but also with smaller molecules with molecular weights of less than 300-350 Daltons. - On several modern, endcapped C18 columns three peaks were found (right-hand chromatogram).
 On strongly polar phases (middle: fluorinated phase, right: very strongly silanophilic phase) the two strong bases (which on the right coelute as one quite narrow peak) could be separated, four peaks are obtained.
On strongly polar phases (middle: fluorinated phase, right: very strongly silanophilic phase) the two strong bases (which on the right coelute as one quite narrow peak) could be separated, four peaks are obtained. - For years an early eluting unknown peak was obtained with many different C18 phases, so too on the polar stationary phase Polaris C18 A (left) and on the hydrophobic, endcapped Discovery C18 (middle),
 It was accepted. First through the use of Primesep C, a C18 phase with additional complexing properties was it possible to show that the first unknown peak was by no means homogeneous. "In the case of unknown samples, testing with truly orthogonal columns is advisable".
It was accepted. First through the use of Primesep C, a C18 phase with additional complexing properties was it possible to show that the first unknown peak was by no means homogeneous. "In the case of unknown samples, testing with truly orthogonal columns is advisable".
- On the two C18 columns LiChrosorb and Reprosil AQ, with pore diameters of 100 Å and 120 Å respectively, five main peaks appear; with Jupiter, also a C18 material but with a pore diameter of 300 Å six main peaks
- "It is advantageous to enable several interaction mechanisms at the same time!"
Several tests with different compounds and under different conditions have shown the following: there are columns which show consistently good selectivity for quite different substance classes ("universal columns"). Only in the case where a column changer cannot be used could such a column, as a second-best alternative, take on the role of a starting universal column for different, unknown samples. Examples of such columns would be LiChrospher C18 or Superspher C18, Purospher/-Star, Nucleosil AB, YMC Pro C18 RS, SMT OD C18, Uptispher UP 5 MM1, XSelect CSH Fluoro-Phenyl.
These materials have particular properties that give them a certain general selectivity for a number of different substance classes.
Brief comments:
Nucleosil AB (25 % C), YMC Pro C18 RS (22 % C) and SMT OD C18 (24 % C): High density, cross-linked, quasi-polymer layer on the surface. This is advantageous for the separation of non-polar/polar components as well as for aromatic compounds in general and especially for steric selectivity.
Purospher: Material endcapped with amino groups, thus polar character. In addition, with 500 m2/g a large specific surface area and with approx. 3 µmol/cm2 a strong coating of the surface.
Uptispher UP 5 MM1: This is a mixed-mode phase with C8 alkyl chains and strong cation exchange groups. Experiments not shown here prove that this material actually does have a large surface diversity.
Primesep C: Es handelt sich dabei um eine Mixed-Mode-Phase mit Alkylketten und einer zusätzlichen Kompexbildung-fähiger Gruppe. Hier nicht gezeigte Experimente belegen, dass dieses Material tatsächlich eine große Oberflächen-Diversität aufweist
LiChrospher C18 and Superspher C18: As older materials they have both acidic and undissociated silanol groups, as well as metal ions in the silica gel matrix. Finally, with 17 - 21 % C, the hydrophobic character is quite pronounced. Such materials offer different interactions to the sample molecules: Hydrophobic, ion exchange, dipole-dipole interactions, via the free electron pair with existing siloxane bonding also π-π-interactions, finally hydrogen bonding and also complex formation via the existing metal ions in the silica gel matrix. The probability for the separation of different molecules is thus given.
XSelect CSH Fluoro-Phenyl; high density, charged groups, silanol groups, electron-rich fluorine atoms, result: here too a large diversity of interactions is possible.
- "Alternative strategy to "A" and "B": "Good", classic C18 column plus subsequent test using one or more completely different column(s) to confirm selectivity (orthogonal test, see above)
Let us assume that a number of rather unproblematic substances are to be separated, e.g. neutral/via the pH value neutralised, weakly polar, uncharged molecules (aldehydes, ketones, weak acids/bases, aromatics with neutral substituents), "simple" isomers. In these cases starting with a classic C18 material would be advisable. Examples of hydrophobic fused core materials could be mentioned: PoroShell 120 EC C18, CORTECS C18, Kinetex EVO C18, Accucore C10, Nucleoshell RP C18. Examples of hydrophobic porous materials: Zorbax Eclipse PLUS C18, XSelect HSS C18, XBridge C18/BEH C18, YMC RS C18, Acclaim C18, Nucleodur Gravity C18. A subsequent orthogonal test with one or two polar columns to confirm selectivity/peak homogeneity would be recommended in any case. The following materials are suitable for this: PFP, EPG, mixed-mode, biphenyl, possibly also 60-80 Å and/or 300 Å materials plus methanol as alternative to acetonitrile, or replace approx. 10 % acetonitrile with tetrahydrofuran.
With which analytes do the differences in selectivity of the phases make themselves least/most noticeable?
Simplified rule:
The smallest differences are noticeable with neutral molecules (possibly neutralized via the pH value) and acetonitrile/water mixtures. The largest differences arise in the case of molecules present in ionic form - e.g. a strong base - and methanol/water mixtures.
Findings from the experiments here: If the components differ in molecular size and polarity ("=O", "OH", aromaticity, position of the functional group etc.), the difference in selectivity of the stationary phases is given by a factor in the alpha values of approx. 3-3.5 (alpha EB/Fl). If, on the other hand, the difference is only a methyl or ethyl group, the factor for the alpha values, taking into account all columns investigated, is reduced to approx. 1.1-1.4 (alpha values Propbenz/Methbenz, propyl-3-hydroxybenzoate/methyl-3-hydroxybenzoate), the phases deliver quite similar chromatograms. With regard to the characteristics of the components to be separated, the differences of the stationary phases come increasingly into effect in this order: ∆ CH2/CH3 group, polynuclear aromatics, planar/non-planar molecules, halogenated substances, isomers, small polar aromatics, strong acids/bases. For the latter, the polar character should of course not be reduced by the pH value and/or the use of acetonitrile, see further above. The following table includes the above said and shows the differences in selectivity between stationary phases that can be expected depending on the type of analyte.
| Type of selectivity | Difference(s) in analytes to be separated | Underlying separation factor alpha | Selectivity range of the phases; factor between the smallest/largest alpha value |
| Hydrophobic selectivity (separation of polar/non-polar components) | ∆: CH2 | α EB/T | 1.14 |
| ∆: CH2 + O | α Propbenz/Ethbenz | 1.17 | |
| ∆: 2 x CH2 + O | α Propbenz/Methbenz | 1.45 | |
| ∆: =O | α Fluoren/Fluorenon | 2.25 | |
| ∆: =O + ∆ Aromaticity + ∆ Molecular size | α EB/Fl | 3.54 | |
| Aromatic selectivity (π-π interactions) | ∆ aromatischec character | α Per/Chr | 1.76 |
| Steric selectivity (Accessibility, π-π interactions) | ∆ spatial arrangement | α Tri/o-Ter | 3.65 |
| Polar selectivity I (hydrogen bonds) | ∆ OH- vs. Alkylgruppe | α Phe/EB | 15 |
| Polar selectivity II (Ion exchange interactions) | ∆ components capable/incapable of ion-exchange, ∆ Charge | α Benz/Phe | Ø 20 |
This table makes it clear why it is necessary to define exactly what kinds of analytes are involved before a statement can be made: "This column is similar to...“ For example, in the case of the separation of neutral/neutralized components different stationary phases behave quite similarly (case 1, small differences in selectivity). With the increase of additional polar interactions or the number of possible mechanisms, the differences become more and more pronounced. They culminate in the case of additional ion exchange interactions and with methanol as organic solvent (case 5, very large differences in selectivity).
Combination of stationary phase and components - what can one expect?
"Same" with same!
- Hydrophobic stationary phase and hydrophobic components: that would be neutral or via the pH value neutralized molecules and phases with a hydrophobic surface, i.e. a minimum of active polar centres, the result: good peak shape, probably good selectivity; the chromatograms look similar, the choice of stationary phase is of secondary importance
- hydrophobic stationary phase and polar components: possibly a good peak shape but poor selectivity (e.g. modern endcapped phases and cis-trans isomers or stronger bases)
- Polar stationary phase and hydrophobic components: probably low retention and also poor selectivity
- Polar stationary phase and polar components: probably good selectivity, the problem here might be long retention times and tailing peaks.
Of course, the selectivity can and should be verified or if necessary improved by modifying the following four factors: eluent composition, type of organic solvent/modifier (acetonitrile, methanol, tetrahydrofuran, methyl-tert-butyl-ether (MTBE), ion pair reagents etc.), ∆ pH value, ∆ temperature.